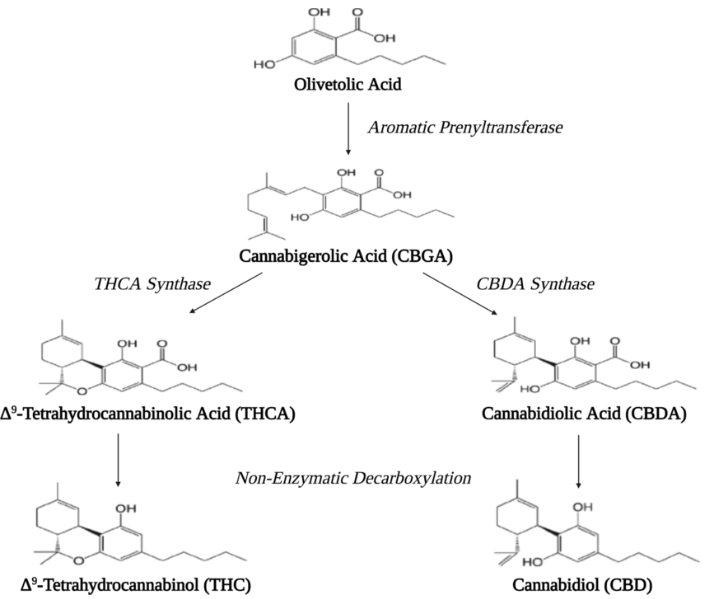
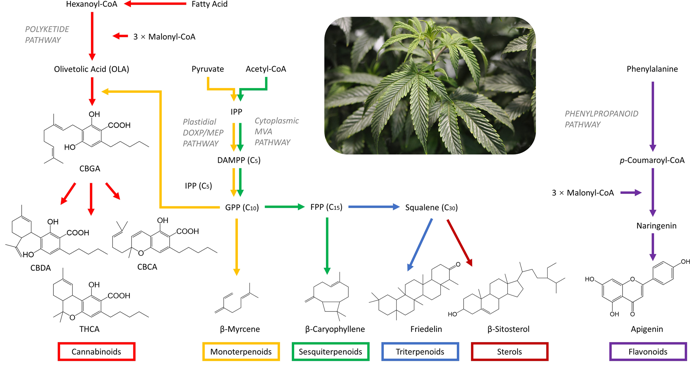
Biosynthesis pathways of cannabinoid, terpenoids, sterols, and flavonoids2,3,4.
Olivetolic Acid (OA) is more than a benzoic acid derivative—it serves as a key biosynthetic intermediate in the pathway of cannabinoids in *Cannabis sativa*. OA condenses with geranyl pyrophosphate through the enzyme Olivetolic Acid Cyclase to form Cannabigerolic Acid (CBGA), the 'mother cannabinoid' that gives rise to major cannabinoids like THCA and CBDA. Because of this central role, OA attracts significant interest in synthetic biology, metabolic engineering, and novel cannabinoid research.
1. Biosynthetic Pathway Role:type III polyketide synthase (PKS) catalyzes the condensation of hexanoyl-CoA and malonyl-CoAs, producing a chain that cyclizes into OA. Subsequently, OA acts as a substrate for prenylation, forming CBGA, which is then converted to THCA or CBDA.
2. Research Relevance: By manipulating OA or its enzymes, researchers can tailor cannabinoid profiles in engineered organisms or plants. OA has also demonstrated a mild anticonvulsant effect in preclinical models, suggesting broader pharmacological potential.
- Synthetic Biology / Metabolic Engineering:** OA serves as a precursor for customized cannabinoid biosynthesis.
- Research Tool:** Substrate or standard in enzymatic and biosynthetic studies.
- Pharmaceutical Exploration:** Potential lead compound for neurological applications.
- Formulation Development:** OA derivatives appear in patents for stabilization and controlled release systems.
For professionals in cannabinoid research, plant biochemistry, or drug discovery, Olivetolic Acid (CAS 491-72-5) is a cornerstone molecule. It represents a bridge between basic biosynthetic processes and applied innovation. Leveraging OA in research could open new doors in understanding cannabinoid pathways, designing analogues, and developing next-generation therapeutics.
Best regards,
Qixi Chemicals – Your trusted partner in fine chemicals & export supply.
Reference
1.Preteroti, M., Wilson, E.T., Eidelman, D.H. et al. Modulation of pulmonary immune function by inhaled cannabis products and consequences for lung disease. Respir Res 24, 95 (2023). https://doi.org/10.1186/s12931-023-02399-1
2.Jin, D., Dai, K., Xie, Z. et al. Secondary Metabolites Profiled in Cannabis Inflorescences, Leaves, Stem Barks, and Roots for Medicinal Purposes. Sci Rep 10, 3309 (2020). https://doi.org/10.1038/s41598-020-60172-6
3.Flores-Sanchez, I. J. & Verpoorte, R. PKS Activities and Biosynthesis of Cannabinoids and Flavonoids in Cannabis sativa L. Plants. Plant and Cell Physiology 49, 1767–1782 (2008).
4.Andre, C. M., Hausman, J.-F. & Guerriero, G. Cannabis sativa: The Plant of the Thousand and One Molecules. Frontiers in Plant Science 7, (2016).
Leave A Message
Scan to Wechat/Whatsapp :

